3 2 Dimethylamino Ethyl 1h Indol 4 Yl Acetate
listenit
Jun 14, 2025 · 5 min read
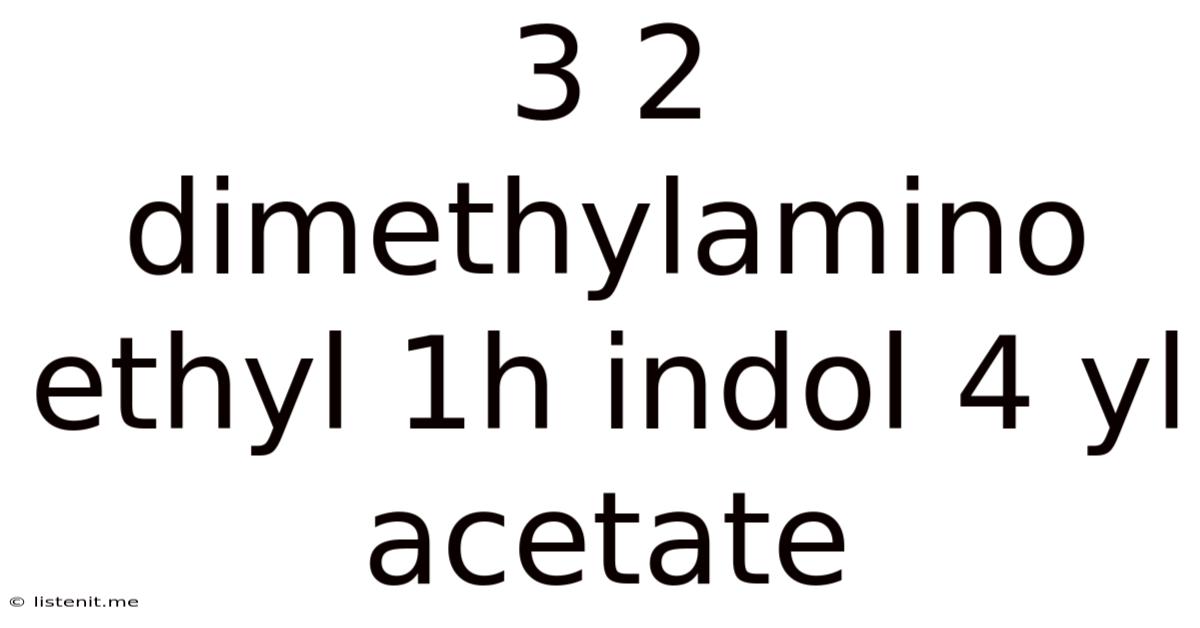
Table of Contents
3-(2-Dimethylaminoethyl)-1H-indol-4-yl acetate: A Deep Dive into its Properties, Synthesis, and Potential Applications
3-(2-Dimethylaminoethyl)-1H-indol-4-yl acetate, a complex organic molecule, presents a fascinating area of study due to its unique structure and potential applications. This comprehensive article will explore its chemical properties, potential synthesis pathways, and promising areas of research and development. While specific applications and research data may be limited in publicly available information due to ongoing research and potential proprietary information, we will explore the fundamental aspects of this compound and its potential based on its structural features and related compounds.
Understanding the Chemical Structure and Properties
3-(2-Dimethylaminoethyl)-1H-indol-4-yl acetate is characterized by its core indole ring structure, modified with a dimethylaminoethyl substituent at the 3-position and an acetate group at the 4-position. This combination of functional groups significantly influences its chemical and potentially biological properties.
The Indole Nucleus: A Foundation for Biological Activity
The indole nucleus, a bicyclic structure containing a benzene ring fused to a pyrrole ring, is prevalent in numerous biologically active compounds. Many alkaloids, neurotransmitters, and pharmaceuticals incorporate this fundamental structure, contributing to their diverse pharmacological effects. The presence of the indole ring in 3-(2-dimethylaminoethyl)-1H-indol-4-yl acetate suggests potential for similar biological activity.
Dimethylaminoethyl Substituent: Impact on Properties
The dimethylaminoethyl side chain introduces a significant level of basicity to the molecule due to the presence of the tertiary amine group. This basicity could significantly influence its interactions with biological systems and its solubility in various solvents. The dimethylaminoethyl group may also impact the molecule's lipophilicity, influencing its ability to cross cell membranes.
Acetate Ester: A Potential Prodrug Strategy
The acetate group at the 4-position functions as an ester. This could be a crucial aspect in the design of the molecule, suggesting it may be a prodrug. Prodrugs are inactive precursors that are metabolized in vivo to release the active pharmaceutical ingredient. The acetate ester may be enzymatically cleaved in the body to reveal a more potent or active metabolite, which would then exhibit its biological effects.
Potential Synthesis Pathways
While the exact synthesis procedures for 3-(2-dimethylaminoethyl)-1H-indol-4-yl acetate might not be widely published, we can infer potential routes based on established synthetic methodologies for similar indole derivatives.
Stepwise Approach: Protecting Groups and Selective Reactions
A potential synthetic approach would involve a stepwise strategy to introduce the substituents onto the indole core. Protecting group strategies are crucial to ensure selective reactions and prevent unwanted side products. A possible strategy would involve:
-
Indole Synthesis: Several methods exist for the synthesis of the indole core itself, such as the Fischer indole synthesis or the Larock indole synthesis. Choosing the optimal method depends on the desired substituents and overall efficiency.
-
Introduction of the Dimethylaminoethyl Chain: This could be achieved via a nucleophilic substitution reaction, possibly using a suitable halide derivative of 2-dimethylaminoethanol.
-
Introduction of the Acetate Group: Acylation of the 4-position of the indole ring can be achieved through an esterification reaction, using acetic anhydride or acetyl chloride under appropriate conditions.
-
Deprotection (if necessary): If protecting groups were used, a final deprotection step would be necessary to yield the desired 3-(2-dimethylaminoethyl)-1H-indol-4-yl acetate.
Optimization and Yield Considerations
The synthesis of this molecule likely requires careful optimization of reaction conditions, including temperature, solvent selection, and reagent stoichiometry. The yield of the final product is a crucial factor to consider for scalability and cost-effectiveness.
Potential Applications and Areas of Research
Given its unique structure and functional groups, 3-(2-dimethylaminoethyl)-1H-indol-4-yl acetate holds potential across various research domains. However, it's crucial to remember that this discussion is based on the structural properties and potential derived from related compounds. Specific pharmacological activity and efficacy require thorough in vitro and in vivo studies.
Potential Pharmacological Activity
The presence of the indole nucleus and the dimethylaminoethyl group suggests potential interactions with various biological targets, possibly impacting neurotransmission, receptor binding, and enzyme activity. Furthermore, the acetate ester's potential to act as a prodrug adds another layer of complexity and possibilities.
Areas for Future Research
Further research is needed to fully elucidate the potential applications of this molecule. This includes:
- In vitro studies: Investigating the compound's interactions with specific biological targets, including receptors, enzymes, and ion channels.
- In vivo studies: Evaluating its pharmacokinetic and pharmacodynamic properties in animal models.
- Toxicity studies: Assessing its safety profile and potential toxicity.
- Structure-activity relationship (SAR) studies: Exploring modifications to the molecule's structure to enhance its potency, selectivity, and efficacy.
Potential Therapeutic Applications (Speculative)
Based on its structure and potential pharmacological activity, speculative therapeutic applications might include:
- Central Nervous System (CNS) activity: The combination of the indole and dimethylaminoethyl groups suggests potential effects on neurotransmission, which could be relevant for treating neurological disorders.
- Analgesic activity: The indole scaffold has been found in several analgesic compounds, suggesting potential in pain management.
- Anti-inflammatory activity: Some indole derivatives show anti-inflammatory properties, which could be of interest for inflammatory diseases.
Disclaimer: This information is for educational purposes only and should not be interpreted as medical advice. The potential applications mentioned are speculative and require rigorous scientific investigation before any conclusions can be drawn.
Conclusion
3-(2-Dimethylaminoethyl)-1H-indol-4-yl acetate presents a fascinating molecule with potential for significant applications. Its unique structure, combining the indole core with a dimethylaminoethyl chain and an acetate ester, hints at interesting biological properties. While extensive research is needed to fully characterize its pharmacological profile and potential therapeutic uses, the molecule warrants further investigation due to its potential in various fields. Further research focusing on its synthesis, structure-activity relationships, and biological effects will be crucial in unlocking the full potential of this intriguing compound. The detailed studies are needed to validate the speculative therapeutic uses and to fully understand its mechanism of action, safety profile, and potential clinical applications. This compound represents a valuable area of research with the potential to contribute significantly to the advancement of medicine and other related fields.
Latest Posts
Latest Posts
-
Black And White Birds In Alberta
Jun 14, 2025
-
How To Change Light Bulb Recessed
Jun 14, 2025
-
How Long To Let Bread Cool
Jun 14, 2025
-
How Long Does Beer Keg Last
Jun 14, 2025
-
How Long For Stain On Deck To Dry
Jun 14, 2025
Related Post
Thank you for visiting our website which covers about 3 2 Dimethylamino Ethyl 1h Indol 4 Yl Acetate . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.